
Molecular complexity is not a term people generally associate with the interstellar medium (ISM). However, more than 200 molecular species have so far been detected in the ISM. These range from the simplest and most abundant molecule, H2, to complex biologically relevant molecules, like the simple sugar glycolaldehyde. The fullerene, C60, is the largest molecule detected in the ISM and molecules containing carbon general dominate the list. In the regions where new stars are born, the molecular complexity is generally high. This indicates that if we want to understand the processes underlying star formation, as well as gain an insight into what chemical species are available during planet formation, then we need to understand interstellar chemistry. A number of the interstellar molecules detected so far have no efficient gas phase formation route under interstellar conditions. Among these molecules are, for example, H2 and methanol. It is therefore generally accepted that these molecules are formed on the surface of interstellar dust grains. We try to gain insight into such interstellar surface reactions by studying them in the laboratory under conditions which mimic the interstellar medium – i.e. we study surface reactions under Ultra High Vacuum, at cryogenic temperatures and on surfaces of interstellar relevance.
Studies reveal that there is potentially a very rich interstellar chemistry involving PAHs, and indeed, when including PAHs in the gas phase chemical reaction networks in dense clouds a strong influence on the abundance of many molecular species is found. In photodissociation regions (PDRs) PAHs are believed to play an important role in the photoelectric heating of the gas, through absorption of stellar far-ultraviolet (FUV) photons, which strongly influence the chemistry. Furthermore, the interaction between PAHs and atomic hydrogen is of interest, due to the potentially important role PAHs may play in hydrogen formation. Our group has conducted laboratory investigations of the catalytic role of neutral PAHs in the formation of small molecules.

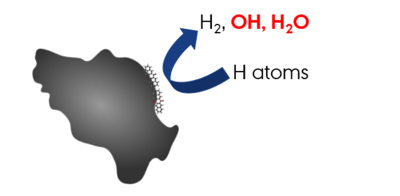
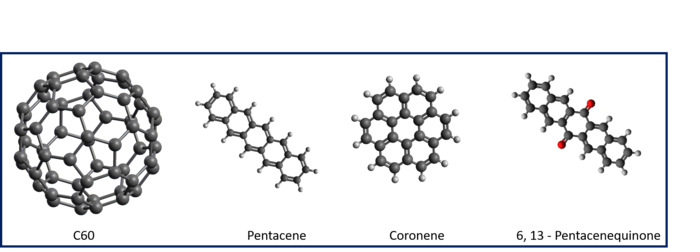
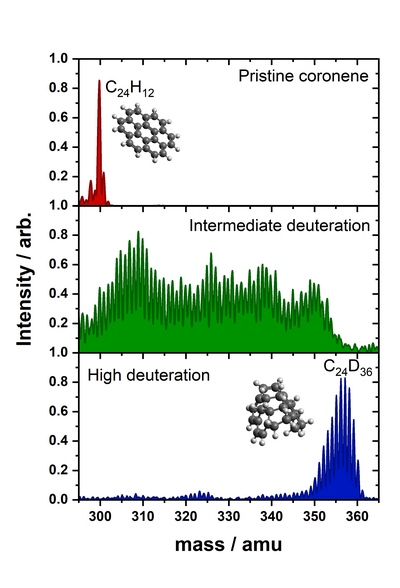
We are particularly interested in the interaction between carbonaceous molecules and hydrogen, especially polycyclic aromatic hydrocarbons (PAHs) and fullerenes. We have previously demonstrated through a combination of density functional theory (DFT) calculations and thermal desorption measurements that the PAH coronene (C24H12) reacts with atomic hydrogen to form superhydrogenated PAH species (HPAHs). Similarly, superhydrogenated PAHs can be formed through interaction with a hydrogenated graphite surface. By performing measurements with atomic deuterium we have further demonstrated that these HPAHs can act as catalysts for forming molecular hydrogen through abstraction reactions. We have similar results for Pentacene and Pentacenequinone. However, since Pentacenequinone is oxygen functionalised, there is scope to study the formation of small molecules apart from molecular hydrogen.
The Surface Dynamics Lab at AU is the coordinating node for the Horizon 2020 Innovative Training Network (ITN) EUROPAH.
The ITN brings together 13 research groups, working across 10 universities and 3 companies around the EU. Our common objective is to understand the role that polycyclic aromatic hydrocarbons play in the physics and chemistry of the interstellar medium.
At the AU node, the SD group is investigating the interaction between polycyclic aromatic hydrocarbon and atomic hydrogen and oxygen. These studies are shedding light on both the formation of superhydrogenated, and other functionalised PAH species, and their role on small molecule formation, for example the formation of molecular hydrogen. This work was initiated under ERC starting grant HPAH.
EUROPAH is a Marie Skłodowska-Curie action funded by the EU under the Horizon2020 framework, grant number 722346
