Trapped molecular ions can be cooled into the millekelvin regime through the Coulomb interaction with simultaneously trapped and laser-cooled atomic ions [Mølhave, PRA (2000)]. The molecular ions are not only cold, but also strongly localized and can be stored for hours. Experiments can be conducted with large samples of thousands of ions or with smaller samples, even single ions. In recent years novel research activities have developed across the world e.g. for studies of (cold) chemistry, high-precision spectroscopy and quantum optics. Here the basics of cold trapped molecular ions and some selected studies by the Ion Trap Group are presented.
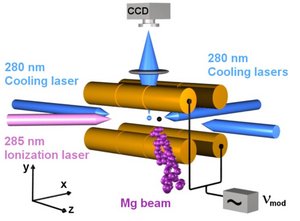
Atomic ions are formed by photoionization of neutral atoms in an effusive beam. The ions are trapped in a linear Paul trap and Doppler laser cooled to ~10 mK temperature. At such low temperatures the ions form an ordered structure, a so-called Coulomb crystal, which is observed by imaging light scattered during Doppler cooling onto a CCD-camera. Trapped molecular ions are sympathetically cooled by laser cooled atomic ions and appear ‘dark’ on the CCD images.
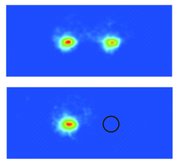
CCD images of two-ion crystals consisting of (top) two laser-cooled atomic ions and (bottom) one-laser cooled atomic ion and one molecular ion (position indicated by black circle).
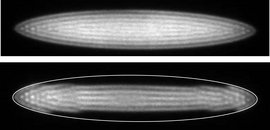
Top: CCD image of a large Coulomb crystal consisting of a few thousands of atomic ions.
Bottom: Molecular ions collect in the ‘dark region’ outside the lighter atomic ions due to the mass dependence of the confining potential.
Molecular ions are formed through reactions with atomic ions, by (photo)ionization of neutral molecules or by fragmentation of other molecular ions.
Examples of trapped species:
MgH+, MgD+, CaH+, CaD+, CaO+,C3H3+, C5H5+, C5H6+, C6H5NH2+
Temperature: 10-100 mK
Number of ions: 1 - thousands
Ion density: ~ 108 cm-3
Ion storage time: hours
Localization: ~ µm3
References: Staanum, Nat. Phys. (2010); Vogelius, PRL (2002)
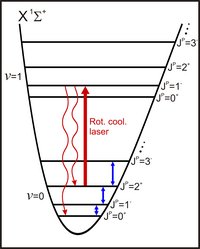
Molecular ions in the ro-vibrational ground state |v=0,J=0>X of the electronic ground state potential can be achieved by optical pumping through the combined action of a PbSe diode laser near 6 µm and the blackbody radiation field
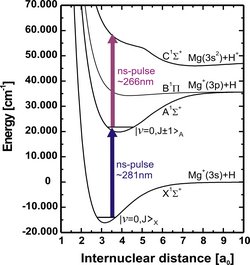
The population in a given rotational state |v=0,J>X is determined as the fractional molecular ion loss following a single combined pulse of 280 nm and 266 nm pulsed light for resonance-enhanced two-photon dissociation.
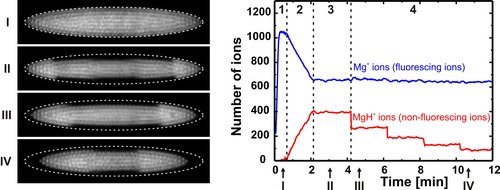
For rotational cooling and population measurements, the following four steps are performed:
1) Formation of a 24Mg+ Coulomb crystal (see Image I)
(2) 24MgH+ ions are formed through the reaction Mg++H2 -> MgH++H by leaking in H2 gas until the desired ion number is reached (see Image II)
(3) Rotational cooling period where the cooling laser near 6 µm is applied
(4) Rotational state selective photodissociation of 24MgH+ ions, here four events of dissociation from |v=0,J=0>X (see Images III and IV recorded after one and four events, respectively)
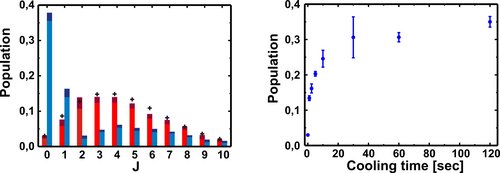
Left: Measured rotational state distributions. The red bars indicate the results without application of the laser cooling beam and the blue bars indicate the distribution after a two-minute cooling period. For comparison, a thermal distribution at 293 K is presented by crosses. When cooling is applied, the ground state population is 37 %, which is equivalent to that of a thermal distribution at 20 K.
Right: The temporal evolution of the population in the ground state during rotational laser cooling.
References: Drewsen, PRL (2004); Staanum, PRATIMS (2010).
When two ions are confined in a harmonic potential, the oscillation frequency of their center-of-mass motional mode depends on the masses of both ions. For one atomic and one molecular singly-charged ion with mass ratio µ, the oscillation frequency ? is given through
![]()
where ?0 is the oscillation frequency for two atomic ions. Thus the molecular ion can be identified from measurements of ? and ?0.
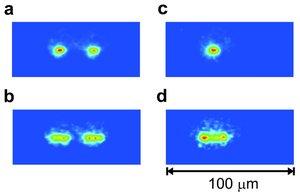
(a) Two 40Ca+ ions at thermal equilibrium in the trap. (b) The same two 40Ca+ ions as in (a), but with a modulated voltage applied to the trap electrodes at a frequency near the resonance frequency ?0.
(c) One 40Ca+ ion and a nonfluorescing 40Ca16O+ ion at thermal equilibrium in the trap. (d) Image of the 40Ca+ ion in (c), when a modulated force at a frequency near the resonance frequency ? is present.
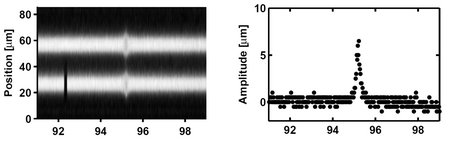
By sweeping the modulation frequency across the resonance frequency in fine steps, a frequency, or mass, resolution of better than 10-3 can be obtained.
Reference: Staanum, PRL (2008).
The sensitive identification technique enables quantitative studies using single ions. We have studied isotope effects in the reaction
Mg+(3p)+HD -> MgH+(MgD+)+D(H)
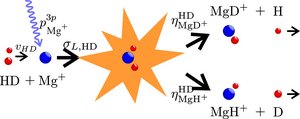
by determining the branching ratio ?MgD+HD/?MgH+HD between the MgD+ and MgH+ channels.
The experiments are conducted by first trapping two Mg+ ions, then leaking in HD gas and finally identifying the reaction product when one of the Mg+ ions has reacted.
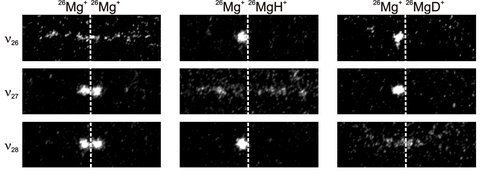
CCD images of two-ion Coulomb crystals with modulation applied at frequencies ?26, ?27 and ?28 for identification of 26MgH+ and 26MgD+ ions. The dashed vertical lines indicate the position of the trap center along the z axis.
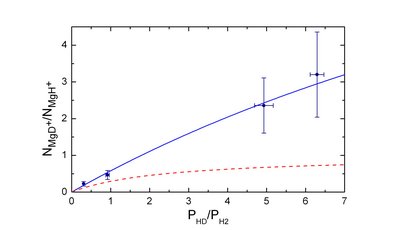
The ratio between the number of formed 26MgD+ and 26MgH+ ions vs the relative pressure of HD and H2. The red dashed line represents the ratio expected in the absence of isotope effects.
Reference: Højbjerre, PRA (2008)
Comparatively complex, or heavy, molecular ions can be trapped and applied for further studies provided the mass-to-charge ratio of the molecular ion is within a factor of ~3 of that of the atomic ion. The present study of trapping and consecutive photofragmentation of aniline ions, cooled by Ca+ ions, demonstrates the advantage of a non-destructive identification technique.
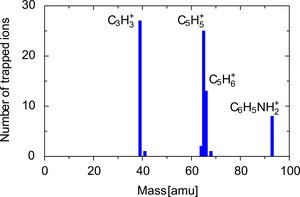
Molecular ion mass spectrum of trapped ions. Other ions than aniline (C6H5NH2+) are observed due to fragmentation (see below) during the initial loading of the trap.
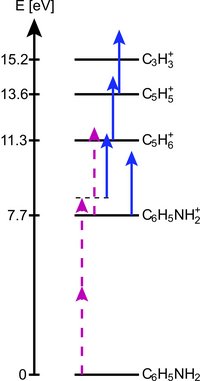
Fragmentation is caused by the laser cooling light at 397 nm and light at 294 nm for ionization of aniline. The energy level scheme indicates the ground state energies of relevant molecular ions relative to the ground state of neutral aniline. The dashed solid arrows indicate some of the photodissociation paths observed in the experiments. The lengths of the arrows correspond to photon energies.
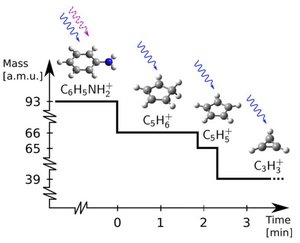
Consecutive photo-dissociation sequence observed in an experiment. The dashed (solid) wiggly arrows represent 294 nm (397 nm) photons responsible for the fragmentations.