New center publication - Rijutha Jaganathan
In this paper we study, experimentally, the interaction of neutral 6, 13 - pentacenequinone and pentacene with H-atoms and compare their reactivity and reaction products using temperature-programmed desorption combined with quadrupole mass spectrometry.
Title: Enhanced reactivity of oxygen-functionalised PAHs with atomic hydrogen - A route to the formation of small oxygen-carrying molecules
Authors: R. Jaganathan, F.D.S. Simonsen, J.D. Thrower, L. Hornekaer
https://www.aanda.org/component/article?access=doi&doi=10.1051/0004-6361/202243312
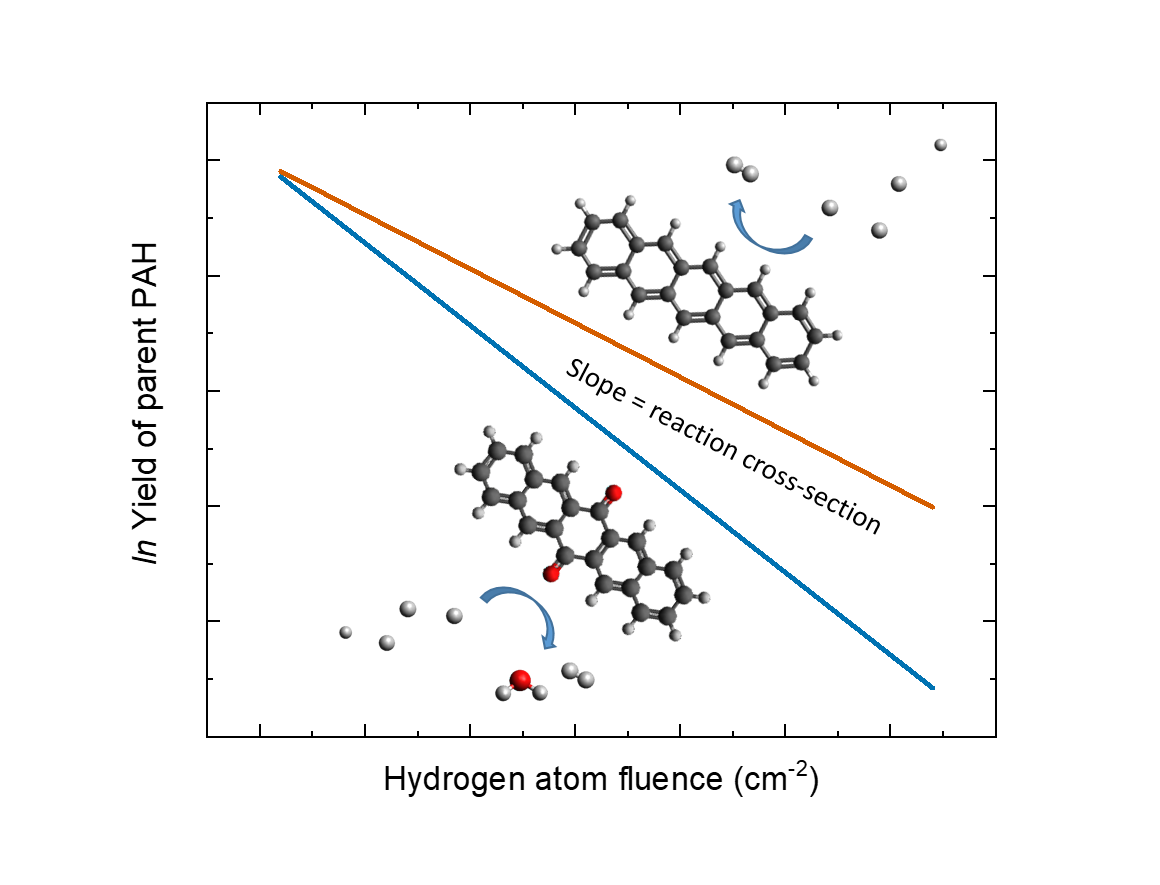
In this paper we study, experimentally, the interaction of neutral 6, 13 - pentacenequinone and pentacene with H-atoms and compare their reactivity and reaction products using temperature-programmed desorption combined with quadrupole mass spectrometry. The main findings are:
- Reaction cross-sections are significantly larger for the oxygen-functionalised species, 6, 13 – pentacenequinone, compared to pentacene.
- Reaction cross-section of pentacene is larger than that of coronene thereby providing experimental evidence for the higher reactivity of zigzag edges
- For both pentacene and 6, 13 - pentacenequinone, hydrogenated species with an even number of excess H-atoms dominate over hydrogenated species with an odd number of H-atoms.
- The end product, after exposure to large H-atom fluences, for both pentacene and PQ is fully superhydrogenated pentacene (C22H36), with little evidence for any remaining oxygen-containing species. This suggests the release of small molecules such as OH and/or H2O by the abstraction of oxygen atoms during hydrogenation, indicating that oxygen-functionalised PAHs can enable the formation of small oxygen-bearing molecules under interstellar conditions.
