Project 1
Imaging spectroscopy of clusters and nanodroplets using EUV radiation
The interaction of small pieces of matter – clusters of atoms or molecules or nanometer-sized droplets – with energetic radiation is complex; there are many ways how a nanoparticle can react to the absorption of a photon. Light can be emitted back from the cluster, an atom in the cluster can be ionized, and energy or charge can be exchanged between the atoms, so that in the end many electrons and ions come out with considerable kinetic energy. Studying these processes is important to get a fundamental understanding of electron correlations, non-adiabatic couplings, and energy transport on the nanoscale. But these processes are also important in nature -- for example where sun light interacts with aerosol particles and induces chemical reactions -- and for technical applications -- for example where EUV light is used for treating surfaces or even living tissue.
In this project we will investigate the interaction of EUV radiation generated by the synchrotron light source ASTRID2 at Aarhus University with clusters and nanodroplets produced in a particle beam in vacuum. To obtain the maximum amount of information about the cluster-light interaction, we simultaneously detect electrons and ions emitted by the clusters in all directions. From the measured energies and emission angles we can learn many details about the initial structure and composition of the cluster, and how it reacts to the light absorption.
Our favorite system is helium (He) nanodroplets – superfluid clusters composed of 100 up to 100 million He atoms. These nanodroplets have unusual properties, including a very low equilibrium temperature (0.4 K), very weak binding of the constituent He atoms (7 K), a homogeneous density distribution, and the ability to pick up “dopant” atoms and molecules which they collide with [1]. Due to their ultra-low temperature, He nanodroplets are superfluid – a peculiar phase which is determined by quantum mechanics. Due to that, dopant atoms but also excited He atoms (He*) and dimers (He2*) are believed to move freely around the droplets. Therefore, several dopants in one droplet readily aggregate to from molecules and clusters, which efficiently thermalize to the ultra-low droplet temperature.
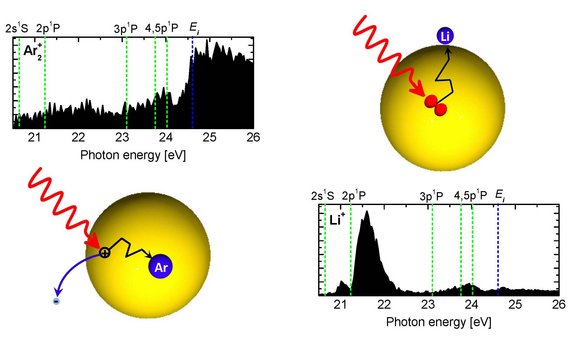
Upon photoexcitation or ionization, He nanodroplets have been found to undergo complex relaxation dynamics, including intra- and interband transitions, fluorescence emission, and ejection of Rydberg atoms and ions. Charge and energy transfer processes, which we have recently studied, include Penning ionization of dopants by He* [2] (see figure above, reproduced from [2]), interatomic Coulombic decay [3,4], and electron-transfer mediated decay – a process where a doubly ionized dopant atom is created from a He+ ion [5]. There are many more intriguing questions related to the back-and-forth transfer of energy and charge in doped He nanodroplets, but also in solid heavy rare gas clusters, and in water and metal clusters.
[1] J. P. Toennies and A. F. Vilesov. Angew. Chem. Int. Ed., 43:2622, 2004
[2] D. Buchta et al., J. Phys. Chem. A, 2013, 117 (21), pp 4394–4403
[3] M. Shcherbinin et al., Phys. Rev. A, 96 013407 (2017)
[4] A. C. LaForge et al., Nat. Phys. 15, 247 (2019)
[5] A. C. LaForge et al., Phys. Rev. Lett. 116, 203001 (2016)
Acknowledgements:
Aarhus Universitets Forskningsfond (AUFF)
Deutsche Forschungsgemeinschaft (DFG), Project MU 2347/10-1
EU support via CALIPSOplus
Project 2, in collaboration with MPI-K in Heidelberg
Helium nanoplasma dynamics
Doped He nanodroplets are widely used as inert, transparent, and cold matrix for spectroscopy of embedded molecules and clusters. However, when exposed to strong laser fields, a few dopant atoms (for example Xe) are enough to “ignite” avalanche-like ionization that turns the whole droplet into a strongly absorbing nanoplasma [5-7]. As a result, the complete He droplet fully ionizes and explodes, see the snapshots from a MD simulation below. Highly energetic electrons, He+, He++, as well as highly charged dopant ions (up to Xe21+) are produced.
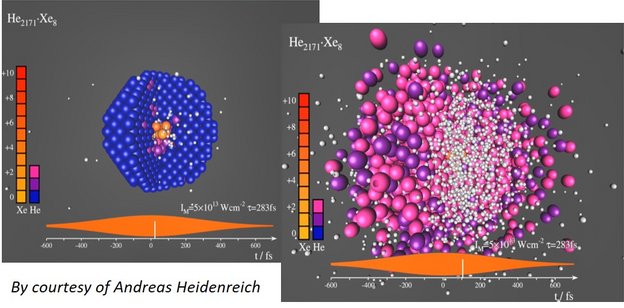
The fundamental questions, which we want to investigate, are:
· How does ignition of the He nanoplasma occur in detail?
· What processes determine the nanoplasma evolution?
· Why is one type of dopant particle better suited than the other for igniting a He nanoplasma?
· What is the role of resonances (collective oscillation of the electron cloud driven by the laser field) when the nanoplasma absorbs light while it builds up and then expands?
· What kind of applications could such nanoplasmas be used for?
We approach these questions experimentally using intense, ultrashort laser pulses, which we shine on a beam of He nanodroplets doped with atoms and molecules of various species. The ejected electrons and ions are detected using imaging techniques, which provide information about the number of electrons/ions ejected, their energy and angular distribution.
[5] S. R. Krishnan et al., PRL 107, 173402 (2011)
[6] A. Heidenreich et al., New Journal of Physics 18, 073046 (2016)
[7] A. Heidenreich et al., Journal of Modern Optics 64, 1061 (2017)
Acknowledgements:
Deutsche Forschungsgemeinschaft (DFG), Projects MU 2347/12-1
Project 3, in collaboration with University of Freiburg
Femtosecond dynamics of doped He nanodroplets
Today, He nanodroplets are mostly used as “nearly ideal” spectroscopic matrix for isolating atoms, molecules, and clusters in an ultracold, weakly perturbing environment [1]. Infrared spectra and, in certain cases electronic spectra, are particularly well-resolved, so they tell a lot about the structure of the embedded particles. Another important motivation for studying He nanodroplets is probing superfluidity in a nanometer-sized system. Several indications have been found that even small He nanodroplets (~100 He atoms) exhibit superfluid behavior. But only very little is known about how such a superfluid system responds in time when it is impulsively excited. And how is the internal motion of a molecule (rotation, vibration, dissociation) altered when it is embedded in a He droplet? To get some insight into this, we study the dynamics of atoms and molecule embedded inside or attached to the surface of He nanodroplets using femtosecond laser spectroscopy.
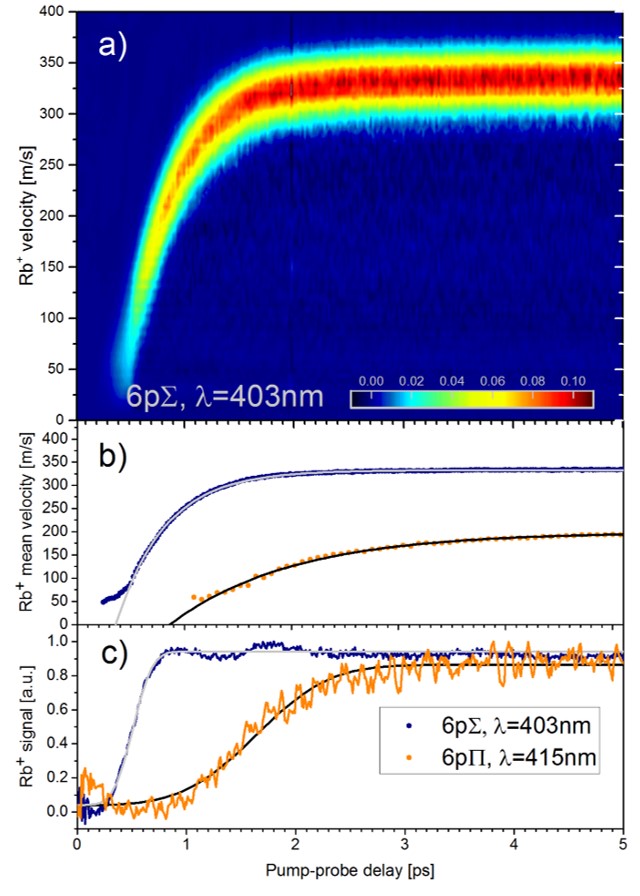
For alkali metal atoms and molecules which stick to the surface of He droplets, we have found that optical excitation induces the desorption off the droplet surface within about 1 up to 100 picoseconds [8]. In contrast, when the atom is ionized and the photoelectron is emitted, the remaining ion sinks into the droplet and becomes strongly solvated. Thus, by applying two time delayed ultrashort laser pulses -- one that excites and one that ionizes -- we can follow the desorption of the atom, and the fall-back dynamics of the ion into the droplet, see figure below (for rubidium, Rb). Other questions, which intrigue us, are: How fast is an atom or ion ejected out of the droplet when it initially sits inside? How does a molecule vibrate inside He droplets, how fast is it damped [9]? How do ions (positive and negative!) form complexes with a number of He atoms around them [10]?
[8] J. von Vangerow et al., Journal of Physical Chemistry Letters 8, 307−312 (2017)
[9] B. Grüner et al., Physical Chemistry Chemical Physics 13, 6816 (2011)
[10] S. Müller et al., Journal of Chemical Physics 131, 044319 (2009)
Acknowledgements:
Deutsche Forschungsgemeinschaft (DFG), Project MU 2347/6-1, 7-1, 9-1
Project 4, in collaboration with University of Freiburg
Cold reactive collisions
Elementary reactions are at the basis of any chemical process. The best way to study them is to have two atoms or molecules collide in free space, at controlled collision energy and with full control of all internal degrees of freedom. In this project we do exactly this: We shoot a cold beam of highly reactive, metastable helium atoms onto target of lithium atoms and detect the reaction products (electrons and ions). To get the best possible control of the collision energy we use a supersonic expansion out of a cryogenic pulsed valve for the helium atoms and a magneto-optical trap for the lithium atoms. We play further tricks to control the quantum states of both helium and lithium atoms. At very low collision energies, we expect the reaction rates to be strongly affected by quantum effects such as quantum rainbows [11] and tunneling resonances. This project has recently been upgraded to detect Penning ionization reactions [12].
[11] M. Strebel et al., Phys. Rev. A 86, 062711 (2012)
[12] J. Grzesiak et al., J. Chem. Phys. 150, 034201 (2019)
Acknowledgements:
Deutsche Forschungsgemeinschaft (DFG), Project MU 2347/3-1