New Center article - Yuan Chen and Ewine van Dishoeck
Title: CoCCoA: Complex Chemistry in hot Cores with ALMA
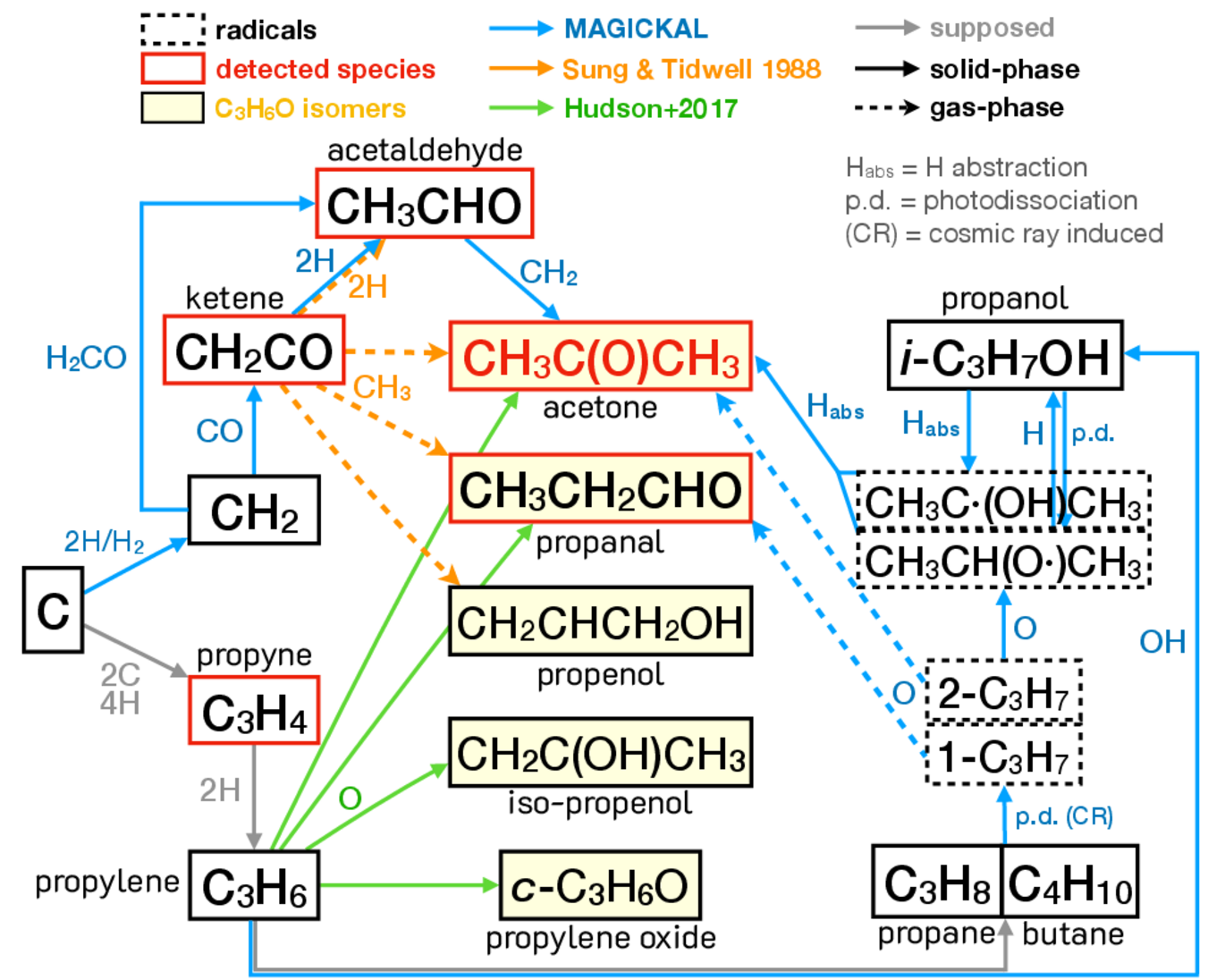
Summary:
Acetone (CH3COCH3) is one of the most abundant three-carbon oxygen-bearing complex organic molecules (O-COMs) that have been detected in space. Recently, acetone ice has been reported as tentatively detected toward B1-c, enabling the gas-to-ice comparison of its abundances. The detection of acetone ice warrants a more systematic study of its gaseous abundances which is currently lacking. Therefore, we conducted systematic measurements of acetone gas in a dozen hot cores observed by the CoCCoA survey and investigate the chemical evolution from ice to gas of acetone in protostellar systems. We fit the ALMA spectra to determined the column density, excitation temperature, and line width of acetone, along with propanal (C2H5CHO), ketene (CH2CO), and propyne (CH3CCH), which might be chemically linked with acetone. We found that the observed gas abundances of acetone are surprisingly high compared to those of two-carbon O-COMs, while aldehydes are overall less abundant than other O-COMs (e.g., alcohols, ethers, and esters). This may suggest specific formation or destruction mechanisms that favor the production of ethers, esters, and ketones over aldehydes. The derived physical properties suggest that acetone, propanal, and ketene have the same origin from hot cores as other O-COMs, while propyne tends to trace the more extended outflows. The acetone-to-methanol ratios are higher in ice than in gas by one order of magnitude, hinting at gas-phase reprocessing after sublimation. There are several suggested formation pathways of acetone (in both ice and gas) from acetaldehyde (CH3CHO), ketene, and propylene (C3H6). The observed ratios between acetone and the relevant species are rather constant across the sample, and can be well reproduced by astrochemical simulations, while more investigations are still needed to draw solid conclusions.
