The energy transfer between two rhodamines, and how to block it
Two working groups in Aarhus and Copenhagen have a recent paper in Angewandte Chemie.
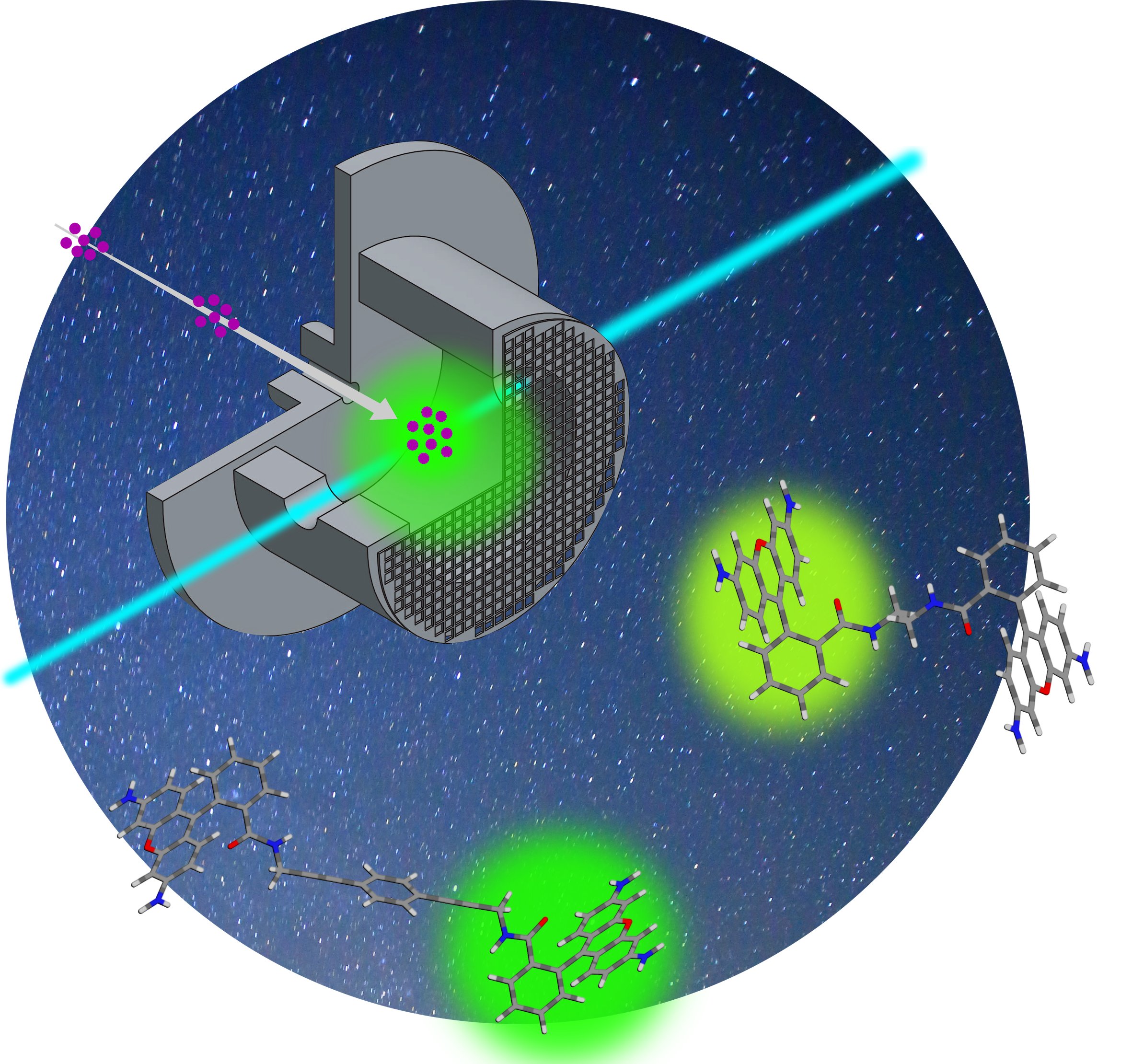
An understanding of the energy transfer between large organic molecules is an absolute nescessity if you wish to come to the bottom of important biological processes. In a paper published on 13 August 2020 in Angewandte Chemie, Steen Brøndsted Nielsen and his research group, in a collaboration with a group of researchers led by Mogens Brøndsted Nielsen, Institute of Chemistry, Copenhagen University describe the processes of energy transfer between two rhodamines each carrying a positive charge.
The rhodamines are a group of fluorescent molecules, amongst other things used as dyes and for tracing currents in liquids utilizing the fluorescense.
The group in Copenhagen has synthezised molecules containing two fluorescent parts, and using the LUNA instrument at IFA the Aarhus group has then investigated the energy transfer from one unit (the donor) to the other (the acceptor) when the donor is subjected to laser light.
Between the two units are either an acrylic bridge (CH2)n or a linear acetylene bridge, -CC-CC. The latter acts as an efficient block towards the energy transfer. Apart from previous studies of these energy transfers, the present study is based on isolated molecules in a gas phase, not allowing other factors like for instance a resolvent influence the processes under study. Only the characteristics of the molecules themselves are looked upon.
The reported screening caused by the acetylene bridge may be important for the energy transport between molecules like e.g. chlorophyll in photo synthetic proteines.
In future studies the molecules will be placed in the new LUNA2 instrument at IFA. In this they will bee cooled to 77K (the temperature of liquid nitrogen) in order to reduce their internal vibrational energy, and thus their flexibility. The expected outcome of this is sharper and nicer spectra, making it easier to identify differences. The new instrument us just about up and running.
To download the full length paper, click on the title below:
Gas-Phase Ion Fluorescence Spectroscopy of Tailor-made
