What is the true colour of chlorophyll, and why might this be important for the debate on energy ressources?
IFA reserarchers Christina Kjær, Bjarke Pedersen, Mark Stockett and Steen Brøndsted Nielsen has reached a further step in isolating the chlorophyll molecule, making a study of its basic spectrum possible. In The Journal of Physical Chemistry for December 2016 the group publishes the new results, being a follow-up to two recent articles in the highly esteemed journal Angewandte Chemie (in 2015 and 2016).
![[Translate to English:] Klorofyl a og b](/fileadmin/site_files/nyheder/blad_og_lup_3.jpg)
The molecules of chlorophyll come in two versions in green plants. They convert sunlight to the energy that plants live by, and this process is far more efficient - up to 95% utilisation - than any of our technological methods for converting energy. If we can lure the way Nature does this, it opens for enormous possibilities, not mentioning the basic scientific satisfaction in understanding of the process.
At present there is a heated debate concerning what mechanism is the prevalent for energy transport between the chlorophyll molecules inside the photosynthesizing proteins. One model describes a process where the energy jumps from one molecule to another, every time to a lower energy state, until the center of reactions is reached (this can be compared to descending af stairway, where the potential energy is reduced discretely for every step downwards you take). In another competing model the energy transport is a non-trivial quantum phenomena. All the chlorophyll molecules are interconnected via the common electron cloud. To settle this debate it is nescessary to study single og very few molecules in order to determine the exited energy levels of isolated chlorophyll, alone in the world (Angew. Chem. i 2015), the coupling between two molecules (Angew. Chem. i 2016) and finally to see how much the energy states are affected by the chlorophyll molecule being in a proteine micro environment (the most recent results).
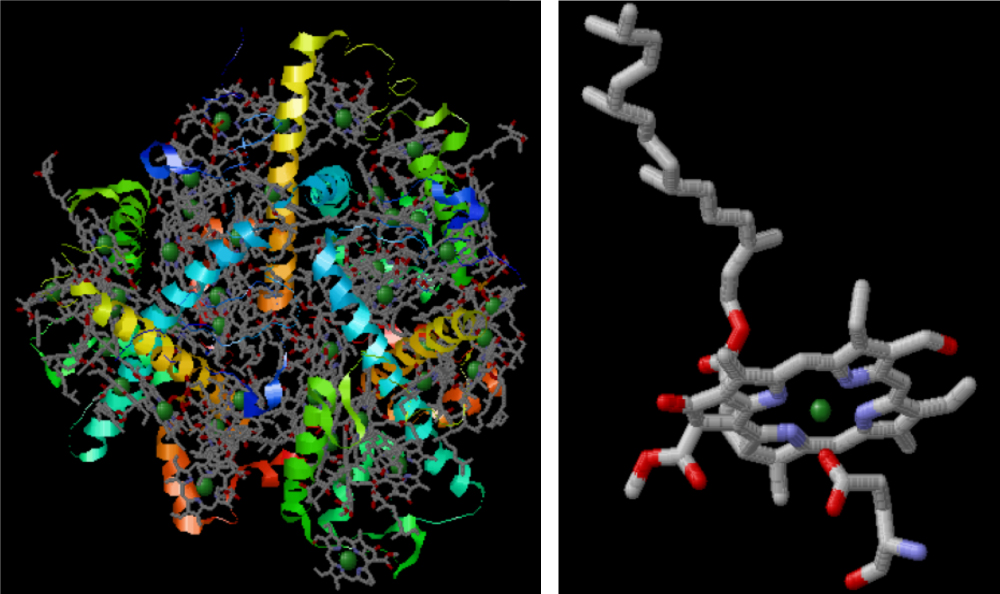
A major problem in the study of chlorophyll is that in Nature one molecule is never alone. Many molcules are always bundled with other compounds into a tangled proteine structure, diffucult to see through and understand. The grey structures in the drawing to the left are alle singel molecules of chlorophyll in the chlorophyll complex of peas. To the right the 3D structure of chlorophyll b is shown.
This is why the IFA group has started "bottoms up", succeeding in isolating the molecules chlorophyll a and b and then attaching an electic charge to each on a molecular location where the spectrum of the basic molecule is almost not affected. Then the charged molecules are sprayed as a thin gas into a vacuum chamber where the spectrographic analysis is done by means of laser light and mass spectrometry.
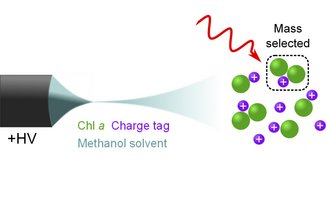
With this method it is possible to determine the basic parameters of the chlorophyll molecules in an (almost) unaffected state. Charged chlorophyll molecules in a methanol solution are sprayed from the left into af vacuum chamber, where molecules of the right mass are selected and radiated by a laser.
Structurally chlorophyll looks a bit like a flye swatter. The most important chemistry takes place in the "head". Adding an electric charge chemically is nescessary in order to be able to handle the molecules in the spraying unit. By attaching the charge to the "handle" one sees that the change in the spectrum is insignificant. With this in place, the researchers have "implanted" another piece of a molecule in the "head", seeing a much larger displacement in the molecular spectrum. This indicates that the important processes in chlorophyll take place here, and the amount of displacement is sufficient to explain the direction of the change that takes place in the chlorophyll spectrum when the molecule is entangled in the larger proteine structures.
"Pure" chlorophyll is coloured more towards blue-green than what we experience when looking at a field of fresh grass. The difference is due to the intercorrellation of the molecules, especially influences on the photoactive "head" of the molecule. This influence will now be studied further.
An important step towards this will be to measure the light output from isolated chlorophyll molecules. This wil then enable the group led by Steen Brøndsted Nielsen to step up to larger and more complex systems, working with a newly finished instrument LUNA (LUminescence iNstrument in Aarhus).
The paper in The Journal of Physical Chemistry can be found here
